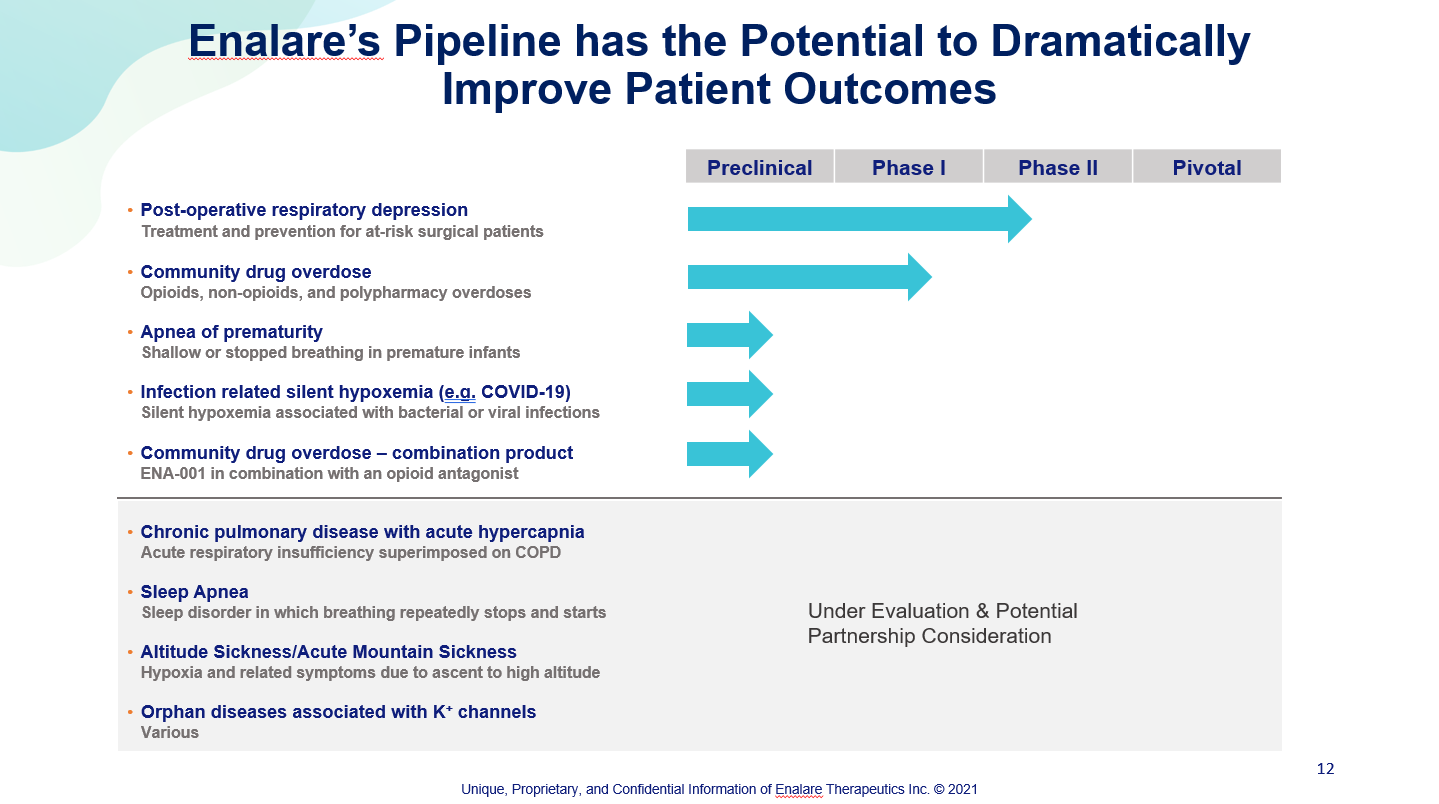
Our lead drug candidate, ENA-001, is a patent-protected new chemical entity (NCE) beginning additional clinical trials during the fourth quarter of 2021. With its novel mechanism-of-action and demonstrated safety and efficacy, it holds the potential of dramatically improving the lives of those impacted by several life threatening conditions including drug overdose, post-operative respiratory depression, and apnea of prematurity. ENA-001 will offer new treatment options for physicians, emergency responders, and care-givers addressing respiratory depression in multiple settings.
Development of Novel Compounds